Description
SPECIFICATIONS
NOTES ON USE
Boric Acid buffers pH changes which may occur during electroforming to prevent localized pH shifts and deposit
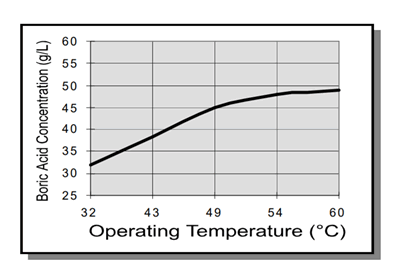
burning. The concentration of boric acid in solution should be maintained at the point of saturation by temperature (see chart on the right). This may be done by using the simple, but effective, method of suspending an anode bag filled with boric acid in solution. This way, boric acid may dissolve in solution as needed. It should be noted that a decrease in bath temperature will result in reduced solubility and subsequent precipitation of boric acid. Though such precipitate is readily removed by filtration, sudden decreases in temperature should be avoided during electroplating to prevent roughness.
ANALYTICAL CONTROL
The required boric acid make – up concentration may be determined by its relationship to temperature.
PRODUCT AVAILABILITY
DisChem Boric Acid is provided in 50 lb. (22.7 Kg.) pails lined with an inner polyethylene bag. Minimum order quantity is 100 lbs.
Boric Acid Titration Guide
Boric Acid – Titration Procedure
A. Required Reagents and Indicators
- 0.1 N Sodium Hydroxide
- D-Mannitol (powder)
- Bromocresol Purple (0.04% aqueous)
B. Procedure
- Sample is to be taken at operating temperature. If allowed to cool before analyzing, re-heat to 140°F to dissolve any precipitated boric acid.
- Pipette 5 mL of heated solution into a 250 mL Erlenmeyer flask.
- Add 2 – 3 drops bromocresol purple indicator.
- Drop wise, titrate with 0.1 N NaOH until solution turns from green to blue. This step neutralizes residual acids other than boric acid. Re-zero burette.
- Add approximately 5 grams D-Mannitol powder, or enough to form a slurry. Solution will turn back to a pale green.
- Titrate, while agitating, with 0.1 N sodium hydroxide to a blue endpoint. Agitate during titration as boric acid dissolves slowly.
C. Calculation
- oz/gal boric acid = (mL 0.1 N NaOH titrated) X (0.164)
- g/L boric acid = oz/gal X 7.5
MANUFACTURERS STATEMENT:
The data contained in this bulletin is believed to be true and
accurate. Optimum results will be obtained when using the
product within the recommended parameters. As final use
of this product is beyond the control of the manufacturer, we
assume no responsibility for misuse of this product, or for
use which may infringe upon third party patents.





